Creativity, Origins, and Ancestors:
What Frog Evolution Can Teach Us
Craig Holdrege
From In Context #35 (Spring, 2016) | View article as PDF
The content of this article is incorporated into a longer monograph, Do Frogs Come From Tadpoles? Rethinking Origins in Development and Evolution. You may also be interested in viewing the video of Craig’s 2017 talk on this topic Where Do We Come From? The Question of Origins and Ancestors in Evolution.
I began a previous article by asking, “Does a frog come from a tadpole?” (Holdrege 2015). The straightforward and seemingly obvious answer is “yes.” Without a tadpole the adult frog could never develop. And of course the tadpole could never develop without adult frogs. In answering the question this way, we are looking at the feature of continuity in space and time, of continuity in the sense world. We can always point to something that is present, formed, and alive “out of which” a next phase of life can develop. And this something is clearly important, because without it nothing could develop further, and also because there is specificity connected with it: out of a salamander embryo a salamander develops and out of a frog embryo a frog develops. What exists in this way in the present is embedded in a long history. There have been thousands upon thousands of generations of wood frogs and bullfrogs. Over time the life history of a given species is repeated — with variations of course —again and again. There is in this sense remarkable stability, with species staying more or less the same for generations.
This is all true. But it also is incomplete and one-sided. It does not encompass a central feature of development and organismic life that only comes into view when we look at the same phenomena differently. For this we need to shift away from focusing on how what already “is” provides the basis for what becomes. Our emphasis is no longer the causal approach that reigns in the biological sciences with its focus on how the past determines the present. Of course, what is antecedent makes possible and also constrains future development. However, it does not provide insight into the special characteristics that arise during development that make an adult frog so different from the tadpole. Since the genomes of the tadpole and the adult frog are virtually identical, it cannot be the genome that creates the differences between them.
The shift in perspective begins when we follow development and organismic life as process and transformation. When we stay in the flow of process itself, and notice the quality of changes that occur, something new shows itself. Instead of focusing on the past as determinant, we see ongoing creation. It is in this sense that we can accurately say that the frog does not come from or develop out of the tadpole. You cannot study the tadpole alone and gain the knowledge that it will develop into a frog. In each generation “adult frog” comes into being through breaking down “tadpole.” When developed, the adult frog actively maintains itself. From this perspective the organism shows itself as creative activity, agency, or being-at-work. The terms we use are not so important; what is important is that we perceive and become vividly aware of the creative, doing-nature of the organism.
Figure 1a. Tadpoles of wood frog (Rana sylvestris)
When we bring these two perspectives together, we see that life plays itself out in the polarity between what has been created and creative activity. (We could also say: between what has become and what is becoming, or between what has been produced and what is producing.) And, as in any true polarity, you don’t find one pole without the other; they are not opposites that can exist separately.
Figure 1b. Mature wood frog (Rana sylvestris)
In a living organism we observe what has been created (already formed substances, structures) and we find creative agency. And just as all forms and structures emerge out of the creative activity of the organism, so does the creative agency remain at work in and through what has already become. We are always dealing with formed life and the formative activity of life.
This double aspect of life is important. The formed life brings a kind of stability and constancy. We would not have anatomy textbooks, nor could we identify and classify groups, if there were not some form of constancy and stability. At whatever level you consider the organism — its DNA, its bones, or the countless generations of wood frogs that are always identifiable as wood frogs—there is stability. But it is essential to realize that such stability is not a static “is.” Every form, structure, or substance is always being actively brought forth and actively maintained. This is being-at-work. All products have been produced; every creature has been created. The active, creative agency of life is always present, not as a thing, but as the process of transformation — of coming to appearance and vanishing.
Every enduring organic structure, every way of being, is a dynamic and persistent pattern in time—a pattern actively and creatively brought forth at every moment. This is the case whether we are speaking of the form of the eye in a single frog or the overall characteristics of a given species.
We are dealing with similar issues when we consider evolutionary development. On the one hand we have the stable existence of life forms over long periods of time, and on the other hand we have the appearance of radically different life forms that never existed previously. The question is: how do we think about the relationship between what existed in the past and what arises as new types of organic forms during the course of evolution?
A standard way of stating the relation would be: all life ultimately evolved from bacteria-like organisms that lived billions of years ago; or, modern humans evolved from ape-or-chimp-like ancestors.
But what do these statements mean? The study of development in the present — as in the example of the metamorphosis of tadpole into frog — can sensitize us to the difficulties that are buried in such statements. It appears to me that just as we can say that a frog cannot be derived from a tadpole, we can also say that humans cannot be derived from earlier primates, or mammals and birds from reptiles, or reptiles from amphibians, or amphibians from fish. In other words, inasmuch as a search for “ancestors” of a given group is looking to find more than temporal antecedents, is looking to find an explanatory “source” or “origin” of a group in the fossil record, it is misguided. It is a search full of expectations that cannot be fulfilled.
My aim in this article is to unpack a more living perspective and to consider the insights and questions it leads to.
Frog Fossil History
In fossils the earth preserves traces in the present of life past. For over 200 years geologists and paleontologists have discovered these fossil traces and striven to decipher and make sense of them. Inasmuch as we can read the fossil record, we gain an opening into the past. The fossil record presents us with a picture of a great diversity in forms of life that have inhabited the earth. It also points to great transformation. Only few organisms that live today, such as horseshoe crabs, have been long present in the fossil record. Most existing species are much more recent appearances. Many groups, such as the dinosaurs, flourished for a span of time and then disappeared. Paleontologists find relationships and discover patterns that suggest that organismic life evolved as part of the evolution of the whole earth.
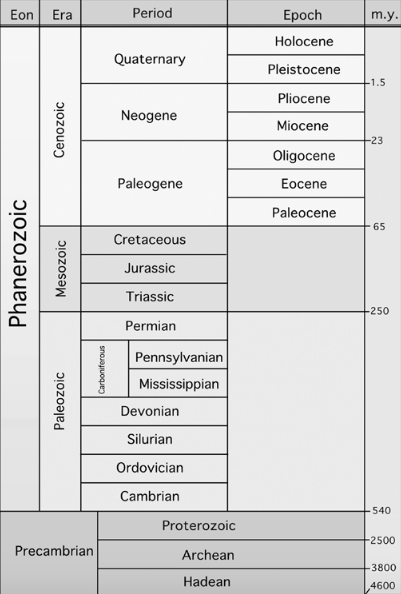
Table 1. Geologic time periods — from most recent (top) to the oldest layers of rock (bottom). m.y. = millions of years (as estimated by measuring radioactive decay in the respective rock layers).
No doubt, the fossil record is woefully incomplete — how few of the organisms that lived in the past actually left traces of their existence! But when one brings the fossil record into relation to all the knowledge one can gain by studying organisms, ecology, and geology in the present day, quite a rich picture emerges. The incompleteness of the picture can serve as a warning to hold it and the conclusions we derive from it lightly and fluidly. Any picture of evolutionary processes needs to be open, mobile and ready to evolve.
Figure 2. Fossil of a large ancient amphibian, Sclerocephalus haeuseri; from the upper Carboniferous (Pennsylvanian) period in Germany. Body length: about 5-6 feet (1.5-1.8 meters). Fossils of frogs appear much later. (State Museum of Natural History, Stuttgart, Germany; Dr. Günter Bechly. https://commons.wikimedia.org/wiki/File:Sclerocephalus_haeuseri,_original_fossil.jpg.)
Figure 3. Well-preserved fossil frog skeleton (Liaobatrachus) found in China, early Cretaceous period. (From Roček et al. 2012.)
Today there are about 4,800 known species of frogs. Each has its particular characteristics, and it is even possible to identify a species on the basis of a few bones. But all frogs have very similar skeletons and since frog skeletal structure is unique among all four-legged vertebrates (tetrapods), a specialist examining fossil bones or imprints can identify whether they belonged to a frog or not. For the most recent periods of the earth’s history one finds fossils that can easily be identified as frogs. For example, wood frog fossils have been found in layers of rock in Nebraska (Pliocene epoch of the Cenozoic era) in which fossils of now extinct animals such as saber-tooth cats and stegomastodons (relatives of elephants) are also found. Frog fossils can be found back into the Mesozoic era (colloquially known as the age of dinosaurs) and can be very well preserved (see Figure 3). In some cases one even finds fossils of tadpoles and partially metamorphosed frogs in one layer (see, for example, Roček and van Dijk 2006).
Figures 4a and 4b (below) show the skeleton of a modern frog and a reconstruction of the skeleton of one of the earliest frog fossils that has been found until now. This fairly complete fossil, given the name Viaraella herbsti, was found in Argentina, and all the bones resemble those of modern frogs. The earliest species found up until now that is considered a frog, Prosalirus bitis, was discovered in Arizona (early Jurassic period of the Mesozoic era). Figure 4c shows a reconstruction based on three specimens that were found (Shubin and Jenkins 1995). With its long hind limbs, the lengthened pelvis, the presence of the urostyle (which is unique to frogs), and the short body, it is clearly a frog. It does have some characteristics in the skull and other parts of the skeleton that distinguish it from modern frogs, but not to the degree that one would think it to be a different kind of animal.
Figure 4. a: Modern frog skeleton (common European water frog, Pelophylax esculentus); b: reconstruction of a fossil frog (Viaraella herbsti) from Argentina, early Jurassic period; c: (below) reconstruction, shown as if jumping, of the currently earliest known fossil frog, Prosalirus bitis,from Arizona, early Jurassic period. (Sources — a: https://commons.wikimedia.org/wiki/File:Rana_skeleton.png; b: Roček 2000, p. 1301; c: Shubin and Jenkins, 1995.)
So it seems that since the early Jurassic period of the Mesozoic era, the basic frog way-of-being, at least as it is manifest in the skeleton, has hardly changed. As a paleontologist who specializes in amphibian evolution writes, “the basic structural scheme of frogs has been maintained without any significant change, which suggests that an equilibrium between function and structure and the mode of life was maintained” (Roček 2000, p. 1295). (The earliest known salamander and caecilian fossils — the other two groups of living amphibians — are also found in Jurassic layers.)
Before the early Jurassic period, no frog fossils have been found. In older rocks (early Mesozoic and Paleozoic eras back to the Devonian period) one finds many fossils of amphibians, all of which have long been extinct. Both larval and adult fossils have been found, mostly in rock formations that geologists believe formed out of sediments at the bottom of ponds and lakes. While in many cases the fossils superficially resemble today’s salamanders, they also have their own array of characteristics that set them apart from the living groups of amphibians (frogs, salamanders, and caecilians). Figure 5 shows a selection of some of these fossil amphibians, most of which were much bigger (1 to 6 meters in length) than today’s amphibians. There is an astounding diversity of forms, as if nature were experimenting with manifold ways to be an amphibian. But it is evident than none resembles a frog. As eminent paleontologist Robert Carroll writes, “despite the great diversity of Paleozoic and early Mesozoic tetrapods that had an amphibious life history, none shows obvious affinities with the three living amphibian orders. This constitutes one of the largest morphological and phylogenetic gaps in the history of terrestrial vertebrates” (Carroll 2000, p. 1270).
Such “gaps” are popularly called “missing links,” and paleontologists have been motivated to continue to search for fossils that would fill the gaps. There is an expectation of some form of continuum, fossils that reveal a closer connection of frogs (and also salamanders and caecilians) to older amphibians. This expectation is based on the view — which Darwin presented in 1859 so forcefully and cogently in Origin of Species — that species evolve gradually out of one another. So paleontologists hope to find fossils that display at least some frog characteristics to bridge the gap between full-fledged frogs and early amphibians. While this hope motivates the search for fossils, it does not generate them, so paleontologists have to live with what they find.
Figure 5. Some examples of the diverse types of amphibian fossils that have been found in the early Mesozoic and Paleozoic eras, before any fossils of the living groups of amphibians (frogs, salamanders and caecilians) are found. Not ordered temporally. (Source: Schoch 2009.)
In the case of frogs, a very few connecting fossils have been found. One is Triadobatrachus massinoti (from the early Triassic period of the Mesozoic era; see Figure 6 and Roček and Rage 2000). Only one specimen has been discovered so far and that was in Madagascar. The skull is quite frog-like in overall shape and in the configuration of the individual skull bones. In contrast, the body is not frog-like: it has a relatively long vertebral column, ribs, a tail, and the hind limbs are short. In other words, the specializations connected with the present-day frog’s leaping mode of locomotion were not present. There is some lengthening of the pelvis and the vertebral column is shorter than in many other amphibians. So the fossil is morphologically “an intermediate between primitive amphibians and anurans [frogs]” (Roček and Rage 2000).
Figure 6. Triadobatrachus massinoti; amphibian fossil from the early Triassic period. Left: Fossil imprint; right: reconstruction. (Sources: imprint from Musee d'Histoire Naturelle, Paris; reconstruction from Roček and Rage 2000.)
Pelvic lengthening and characteristics of the sacrum in another fossil, Czatkobatrachus polonicus, show greater resemblance to frogs, but no skull bones have been found and otherwise it shows little resemblance to frogs. Czatkobatrachus was found in Poland and in somewhat younger layers than Triadobatrachus (Evans and Borsuk- Bialynicka 2009; Rocek and Rage 2000).
Figure 7. a: Doleserpeton annectens; amphibian fossil found in Oklahoma, lower Permian period. Body length: approx. 5.5 cm (2.17 inches); b: partial reconstruction and photo of fossil of Gerobatrachus hottoni, amphibian fossil from the lower Permian period, found in Texas. Body length: approx. 11 cm (4.3 inches). (Sources – a: Sigurdsen and Bolt 2010; b: Anderson et al. 2008.)
Some substantially older fossils from the lower Permian period of the Paleozoic era do not resemble frogs, but they do have a few frog-like characteristics that only a paleontologist with highly specialized knowledge would recognize. One example is in Doleserpeton annectens (from Oklahoma, figure 7a). Its most frog-like characteristics, as in Triadobatrachus, are in the skull: the characteristic (pedicellate) tooth form of modern amphibians (which distinguishes them from most extinct groups of amphibians), the structure of the palate, the stirrup (stapes) in the middle ear, the inner ear, and features of the braincase. Otherwise it resembles other extinct four-legged creeping amphibians and is more like a salamander in overall form than a frog. Gerobatrachus hottoni (from Texas, figure 7b) has, in contrast, a more compact form; it has a broader, more frog-like skull shape and also a shorter spine. It too has pedicellate teeth. Both of these species, unlike the many larger ancient amphibians, are in the size range of modern amphibians.
So in these older layers there are traces of “frogness” but they are present in different species that as a whole were uniquely configured. “Frogness” with all its features does not appear all at once or in only one lineage in the fossil record.
Figure 8. a: Acanthostega, a fossil from the late Devonian period that exhibits both amphibian and fish characteristics; see text. b: Eusthenopteron, a lobed-fin fish fossil from the Devonian period; see text. (Sources — a, top: Clack 2012, p. 165; a, bottom and b: Carroll 1997, p. 300.)
Going back still further, paleontologists continue to find amphibian-like animals. They represent the first four- legged (tetrapod) vertebrates in the fossil record. Some of the earliest tetrapod vertebrates, such as Acanthostega (see Figure 8a), also have fish-like characteristics. These include bony fin rays in the tail, evidence of a lateral line organ, and evidence of internal gills. As paleontologist Jennifer Clack (2006) writes, “If one were to imagine a transitional form between a ‘fish’ and a ‘tetrapod,’ Acanthostega would match almost exactly those expectations.” The detailed anatomy of the four limbs does not suggest that those limbs could support walking on land; more likely they were used as swimming paddles (Clack 2012). This indicates that the seemingly logical and often-presented notion that four-leggedness (tetrapod limbs) in vertebrates developed as an adaptation to living on land isn’t valid. Tetrapod limbs apparently developed first in water and were only in later forms used for moving on land.
The lobed-fin fish Eusthenopteron is a vertebrate fossil from the Devonian period that predates any tetrapods (see Figure 8b). Although clearly an aquatic-dwelling fish, it has certain structures in common with the tetrapods that arise later. Most striking is the internal structure of the fins: the body-near parts of both the pectoral and pelvic fins consist of three bones each; they correspond, in the pectoral fin, to the humerus, ulna, and radius in the forelimb of a tetrapod and, in the pelvic fin, to the femur, tibia, and fibula of the hind limb of a tetrapod. The arrangement of some of the skull bones as well as the presence of internal nostrils are also similar to subsequent tetrapods.
Fishes represent the first vertebrate animals to appear in the fossil record. This is as far back as I want to go—back to the “age of fishes” when manifold types of fishes populated the waters of the earth and no fossils of amphibians, reptiles, birds, or mammals are found.
Forming a Picture of Evolutionary Transformation
From the fossil record we can know that frogs have been a creative presence on earth for a long time. We find frog fossils back into the early Jurassic period of the Mesozoic era. Many species have arisen and passed away since then, but “frogness,” the order “anurans” in scientific terms, has remained present. The diversity of frogs increased over time, and today’s variety, as expressed in 4,800 species, shows the many wondrous ways of being a frog that have evolved.
In earlier layers there are no frog fossils. The first frog fossils have virtually the same proportions and the same skeletal morphology as today’s frogs. So since the early Jurassic, the highly specialized and unique morphology of frogs has remained remarkably constant. Interestingly, something similar happens in many animal and plant groups. As Robert Carroll (1997) writes, “Instead of new families, orders, and classes evolving from one another over long periods of time, most attained their distinctive characteristics when they first appeared in the fossil record and have retained this basic pattern for the remainder of their duration” (p. 167). So paleontologists find periods of fundamental shifts in morphology during which new groups appear and these are followed by long periods of time in which the groups diversify, developing variations on a theme.
But there are also rare and interesting transitional forms. Before there are full-fledged frog fossils, Triadobatrachus and other fossils exhibit some few features — mostly ones in the head — that later all frogs possess. These animals were a far cry from frogs, but if you know frog morphology well, you can see hints of what is to come. Of course, you could never predict, by knowing Triadobatrachus, that frogs would appear later.
What is also typical in the fossil record is that the hints or foreshadowing of what will come later are not manifest in only one type of fossil, but in several. Various elements of what appears later in the new group are manifest in earlier periods, but in different species. Evolutionary scientists often speak in this connection of “mosaic” evolution, since various characteristics appear in different arrangements in different organisms.
In some groups of plants and animals, paleontologists find more transitional forms than in others. But even when a trove of fossils is available, such as in the horse family (Equidae), it is not the case that they line up in a neat series. Rather, there is surprising diversity in the forms that predate modern horses (McFadden 1999). Evolving features appear in different lineages. This is also vividly visible in human (hominid) fossil history; the more fossils we find, the less straightforward the emerging picture of the evolving human form becomes. (See, for example, Lordkipanidze et al. 2013.)
If we consider this feature of the fossil record from a bird’s eye perspective, it is as if we are seeing hints of what is to come spread out in various earlier forms, which then become extinct. Eventually new forms appear, sharing characteristics with various earlier forms but in a new configuration that could never have been predicted on the basis of what came before.
In the Mesozoic and late Paleozoic eras there was a great diversity of amphibians, but only the relatively late appearing frogs, salamanders, and caecilians survived to the present. Among the extinct amphibians, paleontologists find the first four-legged vertebrates (tetrapods) in the fossil record. Just as amphibians today are beings that thrive at the interface of water and land, so the earliest amphibians were four-legged but lived mostly in water. These early tetrapods are preceded in the fossil record of vertebrates by a plethora of ancient fish. Hints of the tetrapod future can (in hindsight) be found in the group of lobed-fin fish. They possessed, as we have seen, a bone structure in the fins that can be viewed as the first beginnings of four-leggedness.
Such phenomena in the fossil record present a picture of major transformation. Manifold new ways of being in the world come to manifestation. This is phylogenetic transformation. Today we find radical organic transformation only in individual development — in embryonic development or in the metamorphosis of the tadpole into the frog. Today frogness “becomes flesh” in each generation of frogs, while during the earth’s history there was a span of time in which frogness became flesh (and sometimes fossilized in rock!) in the stream of then existing vertebrate life. As individual development is an act of creation, so is evolutionary history a record of creativity writ large.
Over the long ages we have been discussing, evolution was occurring with myriad types of organisms. Different ways of being came into appearance — all the kinds of microorganisms, plants, and animals. Since every organism is connected with others in the realization of its life, we need to think of organisms as interpenetrating fields or centers of activity that are in turn influencing and being influenced by the whole ecology of the earth. The fossil record reveals the earth evolving as a whole. It presents traces of a global process of creative transformation.
The Problem of Ancestors
You may have noticed that I have not used phrases such as: “Frogs evolved out of primitive amphibians.” Or: “The first amphibians evolved out of lobed-fin fish.” Why not? First, because such formulations are speculations. While I can observe today how a frog develops “out of ” a tadpole, a similar observation is not given through the fossil record. Second, and more importantly, such formulations overlook at an evolutionary scale precisely what is overlooked today at the scale of individual development when we say that a frog develops out of a tadpole. Yes, a frog does come from a tadpole inasmuch as we are looking to the past (physical continuity of life) and the constraints it presents for further development. But the other side of the coin is that the frog is something new in relation to the tadpole; we can’t understand the adult by examining the tadpole. In every developmental process something new is being created.
When we look at evolution, we can find morphological and other connections between new types of organisms and those that came before them. Frogs are clearly connected in the stream of evolution with early amphibians and, prior to that, ancient fish. We can speculate about possible constraining effects of earlier forms on later ones. But the appearance of new forms shows that such constraints must have been radically overcome in the creation of the new. And that is the case in every group of evolving organisms.
It is well worth noting that in evolutionary research there is a strong drive to identify a particular fossil species that can be labeled as the ancestor of a subsequent group: “Most paleontologists look for ancestors — an ancestor-descendant sequence in which ancestors are assumed to be generalized in a particular character, and the descendants more specialized” (Duellman and Trueb 1994, p. 425). One hopes to find ancestral forms that are general enough to evolve, say, into both frogs and salamanders. But there’s a problem here, which is vividly alluded to by Alfred Romer, a great 20th century paleontologist: “After all an animal cannot spend its time being a generalized ancestor; it must be fit for the environment in which it lives, and be constantly and variably adapted to it” (Romer 1966, p. 25).
All fossils reveal variously specialized animals. If a fossil has characteristics of frogs already, then it is not the ancestor one is looking for, since it is already showing frogness. But if a fossil shows no frogness, then how should we determine whether it is a frog ancestor or the ancestor of some other creature? It can’t be done. As vertebrate paleontologist Robert Carroll writes, “If all relationships are established by the recognition of shared derived [i.e. specialized] features, ancestors cannot be recognized as such because they lack derived traits that are otherwise thought to characterize the group in question” (Carroll 1997, p. 152).
Recognizing the impossibility of determining fossil ancestors, scientists who practice so-called pattern cladistics have set themselves a more modest goal (Brady 1985 and 1994; Williams and Ebach 2009). They simply try to establish relationships between forms: those forms are most closely related to each other that share the greatest number of specialized (“derived”) characteristics. These researchers try to construct dichotomously branching diagrams that express greater and lesser relationship in the context of time. They do not speculate — when they stay true to their principles, which is not always the case — about which forms evolved from which. This is a positive development inasmuch as it restrains speculation and focuses attention on relations and patterns that actually can be observed and discerned by comparing fossils. Problematic, however, is the tendency to dissolve organisms into collections of individual specialized traits and solely on this basis to establish relatedness that is supposed to underlie the evolution of real-life cohesive organisms.
It nonetheless remains a common practice in paleontological and evolutionary (phylogenetic) publications to speak, in relation to closely related groups of organisms, of their “last common ancestor” (LCA). So, for example, in the study of human evolution, the chimpanzee (or its close relative, the bonobo) is considered, in respect to morphology and genetics, the closest living relative to human beings within the animal kingdom. If these two types of beings are closely related today then one expects them to be closely related in evolutionary terms. They must have had a common ancestor. And since the focus is on the continuous stream of physical connectedness through generations, then the conjecture is that there must have been a species that over time differentiated into two different lineages that became, respectively, humans and chimps. If this was the case, then there must have been a “last common ancestor” for humans and chimps. It must have been a real species and theoretically it could be found in the fossil record. Such is the train of thought that motivates the search for ancestors in the fossil record.
The construct of the last common ancestor for any pair of organismal groups seems to be a placeholder for the conviction that there is a branching evolutionary stream that connects all organisms that lived in the past with the living ones. The conviction of the connectedness of all life and its continuity back into the past means that organisms did not evolve out of nothingness, but out of past life forms. This seems reasonable.
But what does “out of ” mean when we restrain the tendency to speculate and refuse to view the past as fully determinative of the future? That is the critical question. If I say that humans evolved out of ape — or monkey — like ancestors (or amphibians out of fish ancestors) and if I mean that humans are further evolved monkeys, or amphibians are further evolved fish, then I am forgetting that specifically human characteristics cannot be derived from monkeys, nor can amphibian characteristics be derived from fish. In both cases new qualities emerged in the stream of evolution that cannot be explained by the past. We miss the creative nature of evolution when we only look at it as a changing and re-arranging of existing material.
There is a strong tendency to conflate building up a picture of the continuity of life in evolution with the question of origins. This tendency manifests itself when evolutionary scientists continue to speak of and to search for ancestors. “An ancestor is, by definition, plesiomorphic (primitive) in every way relative to its descendants” (Cartmill and Smith 2009, p. 62). “Primitive” means, strictly speaking, that it does not have the specialized characteristics of its descendents. But, as Romer pointed out, every species one finds in the fossil record is in its own ways specialized. It is in this sense neither “primitive” nor “generalized,” which is what one implicitly assumes the ancestor to be. So the search for ancestors is the futile search for fully developed organisms that also should somehow indicate that they have potential for further evolution.
The futility shows itself inasmuch as scientists usually end up recognizing that purported missing links or ancestors are too specialized in one way or another to fit the vaguely held notion or expectation of an ancestor. For example, Romer designates an apparent prime candidate for a tetrapod ancestor as being “a bit off the main line” due to its unique specializations (Romer 1966, p. 88). It is altogether clear: biologists work with conflicting and unclear ideas in their search for ancestors that are meant to pinpoint origins.
Thinking About Origins
The quest for ancestors reflects a deep longing to understand origins. It contains, at least implicitly, the question: Where do we come from? The problem is that the quest has been channeled into a vain search for a physical origin that lies in the past. Scientists have been searching for origins in the wrong place and therefore never find what they are looking for. As strange as it may sound, you cannot discover origins by looking to the past alone.
This is the lesson we learn in the phenomenological consideration of organismic development as we observe it today: at any stage of life an organism is both past (what has become) and activity that brings forth something new. The organism as activity or agency is not something in space, some trait or characteristic that you could place next to its skin or stomach. It is the being-at-work in all the features of the organism. It is not something we can directly perceive as an entity. It is what shows itself to the mind’s eye as we follow a developmental process from embryo to adult. It shows itself when we study the way an organism manifests plasticity by “being itself differently” as conditions vary. It shows itself when we compare one type of organism with others and we begin to see its special way of being reflected in all its features.
Evolutionary origins must be creative, capable of bringing the new to appearance. We cannot understand evolution by focusing only on entities that have already been brought forth. Rather, we must follow processes and see connections. The creative and originative is spread out everywhere in the living world and has left its traces in the fossil record. But whether we are able to see it depends on how we look.
We cannot derive the frog from earlier amphibians, as little as we can derive present-day human beings from the many fascinating antecedent hominid forms. Antecedent amphibian fossils indicate the pathways through which frogness comes to appearance in the fossil record, just as studying the transformation of the tadpole shows us how the adult frog comes to appearance. Every new fossil discovery can enrich our growing picture of the story of frog or human evolution and contribute in that sense to our understanding of origins.
But instead of looking for causes in the past — instead of trying to explain evolution through speculative mechanisms — we can shift the focus of research to building up a picture of the immensely creative processes, relations, and patterns that the study of evolution reveals. In one way this is a much more modest undertaking than the attempt to explain our origins as contemporary evolutionary science does. But this undertaking is at the same time demanding. It calls for recognizing and holding back speculation; it calls for our thinking to stay close to the phenomena and to glimpse the reality speaking in the patterns and connections. A deeper understanding of evolution will evolve to the degree that human consciousness evolves. Gathering more facts can be important, but developing our minds to allow more to reveal itself within the field of facts is even more essential.
With the evolutionary appearance of humans on earth and subsequent historical and cultural evolution, beings have arisen who are in a position to consider their own evolution and the evolution of the whole planet. We can study and ponder the evolution of all our fellow creatures on earth and of the earth itself. This is a characteristic that we do not find in the rest of organic life. It is a unique quality of human evolution that we arise as beings who can study and begin to understand, through thoughtful observation and contemplation, the evolving world of which we are a part and in which we participate.
This simple fact has implications that are all too easily overlooked. They have to do with origins. We can only study something that we have a relation to. If something were totally foreign, so that our senses and our mind could find absolutely no relation to it, then it would not exist for us. But we can engage with and study all life on earth. As different as we are from bacteria, mosses, or dragon flies, we do perceive them. We find characteristics that we have in common with them and many others that we don’t share. All this is evidence of the fact that we are deeply and broadly connected with the totality of life on earth. This means that bacteria, plants, and animals are part of us. Certainly, in one sense we are separate beings, but as living and thinking beings we encompass all other life.
We speak today often so glibly of the interconnectedness of all things, imagining separate entities and processes that are connected as in a web or network. But once we begin to understand interconnectedness more deeply, we realize the limitations and misleading nature of an image that begins with separateness and only secondarily establishes relations.
From the perspective of the fossil record human beings as a discrete species are a relatively late appearance. But we can also say that in considering the development of life on earth we are considering our own development. We are intimately connected with the originative forces of evolution.
References
Anderson, J. et al. (2008). “A Stem Batrachian from the Early Per- mian of Texas and the Origin of Frogs and Salamanders,” Nature vol. 453, pp. 515-18. doi:10.1038/nature06865.
Brady, R. H. (1994). “Pattern Description, Process Explanation, and the History of the Morphological Sciences.” Originally published in: Interpreting the Hierarchy of Nature: From Systematic. Available online at https://www.natureinstitute.org/ronald-h-brady
Patterns to Evolutionary Process Theories, edited by L. Grande and O. Rieppel, pp. 7-31. San Diego CA: Academic Press, 1994.
Brady, R. H. (1985). “On the Independence of Systematics,” Cladistics vol. 1, pp. 113-26. Available online at: https://www.natureinstitute.org/ronald-h-brady.
Carroll, R. L. (1997). Patterns and Processes of Vertebrate Evolution. New York: Cambridge University Press.
Carroll, R. L. (2000). “The Lissamphibian Enigma,” in Amphibian Biology, edited by H. Heatwole and R. L. Carroll, pp. 1270-73. Chipping Norton, Australia: Surrey Beatty and Sons.
Cartmill, F. and F. H. Smith (2009). The Human Lineage. Hoboken NJ: Wiley-Blackwell.
Clack, J. A. (2006). “The Emergence of Early Tetrapods,” Palaeogeography, Palaeoclimatology, Palaeoecology vol. 232, pp. 167-89.
Clack, J. A. (2012). Gaining Ground (second edition). Bloomington, IN: University of Indiana Press.
Duellman, W. E. and L. Trueb (1994). Biology of Amphibians. Baltimore MD: The John Hopkins University Press.
Evans, S. E. and M. Borsuk-Bialynick (2009). “The Early Triassic Stem-Frog Czatkobatrachus from Poland,” Palaeontologica Polonica vol. 65, pp. 79-105.
Holdrege, C. (2015). “Do Frogs Come from Tadpoles?” In Context #33, pp. 13-18. Available online: https://www.natureinstitute.org/article/craig-holdrege/do-frogs-come-from-tadpoles
Lordkipanidze, D. et al. (2013). “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo,” Science vol. 342. pp. 326-31. doi:10.1126/science.1238484.
McFadden, B. (1999). Fossil Horses. Cambridge: Cambridge University Press.
Roček, Z. (2000). “Mesozoic Anurans,” in Amphibian Biology, edited by H. Heatwole and R. L. Carroll, pp. 1295-331. Chipping Norton, Australia: Surrey Beatty and Sons.
Roček, Z. and J.-C. Rage (2000). “Proanuran Stages,” in Amphibian Biology, edited by H. Heatwole and R. L. Carroll, pp. 1283-94). Chipping Norton, Australia: Surrey Beatty and Sons.
Roček, Z. and E. van Dijk (2006). “Patterns of Larval Development in Cretaceous Pipid Frogs,” Acta Palaeontologica Polonica vol. 51, pp. 111-26.
Roček Z. et al. (2012). “Post-Metamorphic Development of Early Cretaceous Frogs as a Tool for Taxonomic Comparisons,” Journal of Vertebrate Paleontology vol. 32, vol. 1285-92.
Romer, A. (1966). Vertebrate Paleontology. Chicago: University of Chicago Press.
Schoch, R. (2009). “Evolution of Life Cycles in Early Amphibians,” Annual Review of Earth and Planetary Sciences vol. 37, pp. 135-62.
Shubin, N. H. and F. A. Jenkins (1995). “An Early Jurassic Jumping Frog,” Nature vol. 377, pp. 49-52.
Sigurdsen, T and J. R. Bolt (2010). “The Lower Permian Amphibamid Doleserpeton (Temnospondyli: Dissorophoidea), the Interrelationships of Amphibamids, and the Origin of Modern Amphibians,” Journal of Vertebrate Paleontology vol. 30, pp. 1360-77.
Williams, D. M. and M. C. Ebach (2009). “What, Exactly, is Cladistics? Rewriting the History of Systematics and Biogeography,” Acta Biotheoretica vol. 57, pp. 249–68. doi:10.1007/s10441-008-9058-5.